Poster Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2016
Pembrolizumab Induced Diabetes – A Case Series (#296)
Introduction:
Autoimmune endocrinopathies have been well documented in patients treated with immune check-point inhibitors for cancers. Autoimmune diabetes associated with the humanised monoclonal antibody against programmed death 1 (PD-1), Pembrolizumab, has been described in case reports, although its incidence in pooled results for melanoma and NSCLC trials was low (0.1%). In a short period of using this medication we have seen three additional cases of Pembrolizumab-related diabetes. This communication summarizes their presentation and management.
Case reports
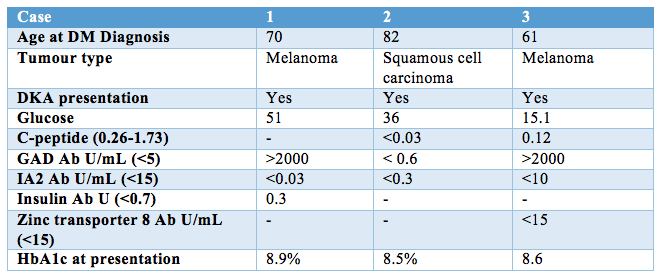
All three cases presented to hospital with diabetic ketoacidosis and required insulin therapy (Table 1). Cases one and three were found to have strongly positive glutamic acid decarboxylase (GAD) antibodies, and all had undetectable protein tyrosine phosphatase-like protein (IA-2) antibodies. Case one had a significant background of rheumatoid arthritis and common variable immune deficiency. On presentation, case one was diagnosed with concomitant immune hepatitis treated with high dose intravenous glucocorticoid, and required a prolonged insulin infusion in this setting. Case 2 received concurrent 5-FU chemotherapy involving intermittent oral steroids, which was associated with brittle and unpredictable glycaemia. Case 3 developed an immune-mediated skin rash and thyroiditis and had a known pancreatic metastasis. Treatment response has necessitated the continued use of Pembrolizumab in all three cases, and all remain on basal bolus insulin therapy several months following diagnosis.
Conclusion:
Pembrolizumab-induced diabetes is a serious and likely irreversible treatment complication and is often precipitated or exacerbated by the use of high dose glucocorticoids for treatment of concomitant adverse immune reactions. Early recognition and prompt treatment with insulin is required to prevent life threatening diabetic ketoacidosis. Endocrinologists and Oncologists need to be aware of this ‘new’ form of diabetes associated with cancer treatments.
Table 1.