Oral Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2016
Effects of linagliptin on glycemic control and albuminuria in type 2 diabetes - the MARLINA–T2D™ trial (#62)
Introduction: Previous evidence suggests that the dipeptidyl peptidase-4 inhibitor linagliptin may exert anti-albuminuric effects beyond glycaemic control.
Objective: MARLINA–T2D™ (NCT01792518), a multicenter, double-blind, placebo -controlled clinical trial, investigated glycemic and renal effects of the DPP-4 inhibitor linagliptin (LINA) in patients with type 2 diabetes, albuminuria and eGFR ≥30 mL/min/1.73m2.
Methods: In total 360 patients with type 2 diabetes (HbA1c 6.5-10%) and persistent albuminuria (urinary albumin-to-creatinine-ratio [UACR] 30–3000 mg/gCr) despite stable background of single renin-angiotensin system blockade were randomized to LINA (n=182) or placebo (n=178) for 24 weeks. Primary glycemic and key secondary renal surrogate endpoints were HbA1c and UACR change from baseline over 24 weeks, respectively.
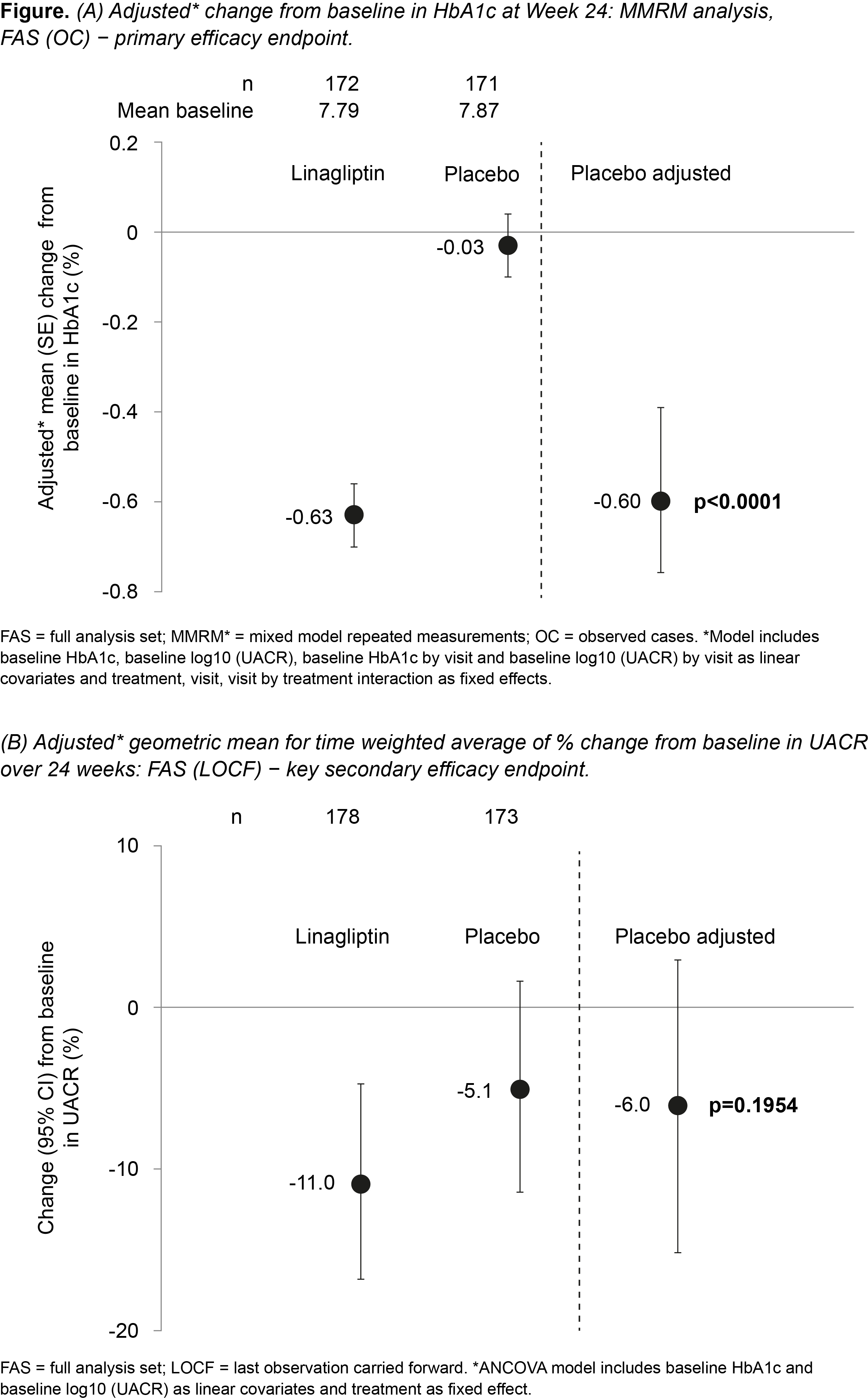
Results: Overall mean baseline (SD) HbA1c and geometric mean (gMean) UACR were 7.8 (0.9) % and 126 mg/gCr (microalbuminuria 73.7%; macroalbuminuria 20.3%), respectively. At week 24, placebo-adjusted mean HbA1c change from baseline was -0.60% (95% CI -0.78, -0.43; p<0.0001; Fig 1A). Placebo-adjusted gMean for time weighted average of % change from baseline in UACR over 24 weeks was -6.0% (95% CI -15.0, 3.0; NS; Fig 1B). LINA was well tolerated with a renal safety profile consistent with previous clinical trials.
Conclusion: LINA significantly improved glycemic control without a significant effect on UACR. Whether a renoprotective effect of LINA could emerge from chronic intervention in more advanced diabetic kidney disease is currently being explored in the ongoing CARMELINA™ trial.